Helpful Downloads

Get ready for your LUMRYZ journey
Once your healthcare provider prescribes LUMRYZ, it's helpful to have a clear picture of what steps should come next. This timeline provides an overview of the typical stages of your journey with LUMRYZ.
 Download Timeline PDF
Download Timeline PDF
Use this tool to help you and your healthcare provider have productive conversations about your narcolepsy and sleep patterns
Community Resources
Make connections with our LUMRYZ Mentor Program

- Chat 1:1 over the phone with someone who is living with narcolepsy and treating with LUMRYZ
- Discuss narcolepsy, LUMRYZ, RYZUP Support Services, how to stay connected, and more

Get matched with a mentor
Call 1-888-209-5105 to be connected with a representative or click to learn more.

LAUREN DISCUSSES THE NARCOLEPSY COMMUNITY
“I think that being able to connect with other people that are going through a similar experience definitely made me feel less alone and empowered me.”
— Lauren T., living with narcolepsy and treating with LUMRYZ
Lauren was compensated by Avadel Pharmaceuticals. Individual results may vary.
Frequently Asked Questions
Select a question below to see the answer
You have 2 dedicated RYZUP™ Support Services Nurse Care Navigators (NCNs) supporting you every step of the way, each with a different focus:
- One NCN is focused on getting you started with RYZUP Support Services, including assisting you with starting RYZUP Support Services, and handling your insurance benefits investigation and financial assistance
- One NCN specializes in LUMRYZ and connects you to support and educational resources you may need at every step of your treatment journey
Not sure who to contact? Just call the main RYZUP Support Services number at 1-844-485-7636, and we’ll direct you to the right person.
Insurance and Financial Assistance
If you are ever unclear on next steps with insurance and benefits investigation, finding financial assistance, prescription shipments, or other questions about starting LUMRYZ, you can call your NCN for support.
No, you can remain on your twice-nightly oxybate until the insurance coverage process for your LUMRYZ prescription is complete.
If you are starting LUMRYZ, you may be eligible for free medication if there is a denial or delay in insurance coverage through the LUMRYZ Quick Start program.* Your NCN will work with your healthcare provider to determine if you are eligible for this or other financial assistance programs for LUMRYZ.
*Applies only to eligible, commercially insured patients.
Your dedicated NCN and RYZUP Support Services will work directly with your healthcare provider’s office to connect you with financial assistance you may quality for, including:
-
Co-Pay Assistance
Your co-pay could be as little as $0 if you have commercial insurance.* -
LUMRYZ Quick Start
Patients new to LUMRYZ may be eligible for free product if there is a denial or delay in insurance coverage.† -
LUMRYZ Bridge Program
Patients already on LUMRYZ may be eligible for free product if there is an interruption in coverage due to insurance.† -
Patient Assistance Program (PAP)
Treatment is available free of charge to eligible patients who are uninsured or underinsured.‡
You can ask your NCN team about available support services at any time during your treatment experience.
*This offer is valid only for patients who have commercial insurance. Offer not valid for patients enrolled in Medicare, Medicaid, or other federal or state healthcare programs. Additional terms and conditions apply. Download the full terms and conditions of the co-pay program.
†Applies only to eligible, commercially insured patients.
‡PAP application required. Patient must meet certain financial and other criteria.
If you lose your insurance coverage or it changes, RYZUP Support Services will receive this status update.
Your NCN will automatically contact your healthcare provider to discuss applicable financial assistance options.
If you expect a change in your insurance or have questions about your insurance status, you can discuss that with your NCN at anytime.
Your NCN will work with you and your healthcare provider to evaluate your eligibility for PAP at the end of each calendar year.
Yes. It is necessary to speak with your specialty pharmacy each time you refill your LUMRYZ prescription as part of the LUMRYZ REMS program and requirements.
LUMRYZ can only be filled through specialty pharmacies in the LUMRYZ REMS program. At each refill, the specialty pharmacy needs to confirm what other treatments you may be taking to ensure safe dosing.
Once you and your healthcare provider find the LUMRYZ dose that is right for you, consider talking to them about adjusting your prescription to refill every 30 days, rather than every 7 days.
REMS, Risk Evaluation and Mitigation Strategy.
Check for any recent communications from your specialty pharmacy about tracking information for your delivery. It may be sent through a shipping partner like FedEx® or UPS®.
Your specialty pharmacy may need to confirm with your healthcare provider if there have been changes to your treatment plan or changes to other medications you are taking. This can sometimes delay delivery.
Tip: If you think you might not be home to receive and sign for your delivery, talk with your specialty pharmacy about authorizing another adult to sign for your LUMRYZ. You can also talk to them about shipping to an alternative address—such as a FedEx or UPS Store—if you are not able to be home.
Your RYZUP Support Services NCN is available if you have questions or feel unsure about next steps.
You can reach your NCN at 1-844-485-7636 from 8:00 AM to 8:00 PM Eastern Time, Monday through Friday.
Tip: If you think you might not be home to receive and sign for your delivery, talk with your specialty pharmacy about authorizing another adult to sign for your LUMRYZ. You can also talk to them about shipping to an alternative address—such as a FedEx or UPS Store—if you are not able to be home.
Taking Your LUMRYZ Dose
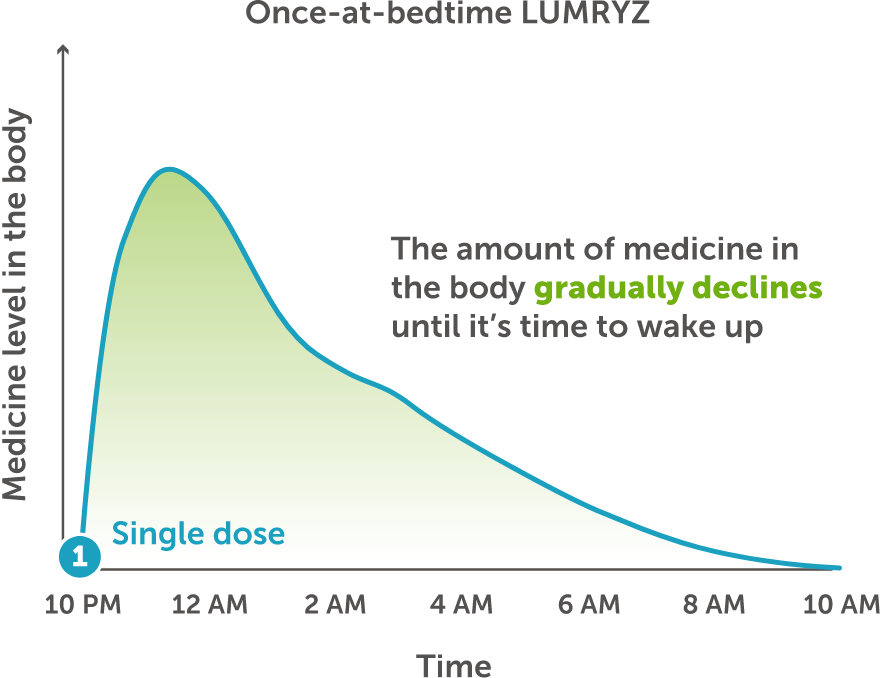
With LUMRYZ, you take 1 premeasured packet, once at bedtime. See the full Instructions for Use for how to prepare, shake, and take LUMRYZ.
Watch this video for a step-by-step guide or download the LUMRYZ Dosing Guide for an offline resource.
After following the full Instructions for Use to prepare your LUMRYZ dose, you may notice the mixture does not fully dissolve in water and has a gritty texture with a salty taste.
This is because the unique formulation is designed to release medicine into the body while you sleep—which is what makes LUMRYZ a once-at-bedtime oxybate treatment.
Titration is the process of allowing the body to adjust to a new medicine by starting with the smallest dose—then going up a dose, slowly over time, and possibly going back down a dose to try and find a stable dose that balances effectiveness and tolerability. It took 3 to 13 weeks for this to occur in the LUMRYZ phase 3 clinical trial.
Because everyone responds to treatment differently, there is no one-size-fits-all approach to LUMRYZ. Your healthcare provider will adjust your dose based on how you are feeling.
It’s important to share all of your treatment experiences with your healthcare provider during the titration process so you can work together to find the best treatment fit for you.
Treatment and Expectations
Everyone responds to treatment differently. In the clinical trial for LUMRYZ, some participants saw significant symptom improvements as early as week 3 while others saw symptom improvements at week 13* after titrating to a higher dose.
It may be helpful to keep track of how you're feeling with LUMRYZ in a symptom journal. This can help you and your healthcare provider with conversations about how treatment is working.
*The LUMRYZ double-blind, placebo-controlled clinical trial included participants with narcolepsy treated with LUMRYZ (n=107). The results measured at week 3 (n=88), week 8 (n=77), and week 13 (n=69) showed daytime symptom improvement of participants on the 6-g, 7.5-g, and 9-g doses of LUMRYZ, respectively. Individual results may vary.
In a clinical trial, the total sleep time did not change from when the trial started to the end of the trial. LUMRYZ participants slept for approximately 7 hours at the beginning and end of the trial.
People with narcolepsy generally sleep the same amount of time at night as people without narcolepsy, but most people with narcolepsy don’t have good quality sleep. Speak to your healthcare provider about your sleep as well as how you feel when you wake up the following day.
The most common side effects reported by adult participants in the clinical trial were nausea, dizziness, bedwetting, headache, and vomiting.
In the clinical trial for LUMRYZ, side effects typically occurred when participants started a new dose. Generally, the side effects then declined over time while staying on the same dose.
The safety of LUMRYZ for the treatment of cataplexy or excessive daytime sleepiness in pediatric patients 7 years of age and older with narcolepsy was determined in a well-controlled trial of immediate-release sodium oxybate.
The most common side effects in children include nausea, bedwetting, vomiting, headache, decreased weight, decreased appetite, dizziness, and sleepwalking.
Explore the side effects over time in the clinical trial for LUMRYZ.
Everyone responds to treatment differently. Your healthcare provider can help guide your treatment. It’s helpful to share how you’re feeling and speak up about any symptoms as soon as you notice them. Your healthcare provider can help you determine whether your dose needs to be changed or if treatment should be continued.
You and your healthcare provider can work together to understand what to do if you are experiencing side effects.
LUMRYZ contains a unique blend of granules that work in 2 ways:
- Immediate-release granules start working as you fall asleep.
- Controlled-release granules start working later so you don’t have to wake for a second dose.